Abstract
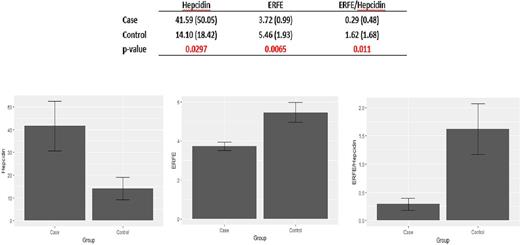
Transfusional iron (Fe) overload is not rare among patients with sickle cell disease (SCD) and can lead to significant morbidity and even mortality. We previously reported that the prevalence of Fe overload among 635 adult SCD patients followed at our Center was 12%, and that the majority (80%) resulted from episodic, mostly unnecessary transfusions in the outlying hospitals (Son et al, 2013). We also showed that the Fe-regulatory peptide, hepcidin was appropriately upregulated in Fe overloaded SCD patients compared to those without Fe overload, and found no difference in levels of inflammatory markers (hsCRP and IL-6) as well as GDF15 between the two groups (Mangaonkar et al, 2014). Recently, a glycoprotein hormone produced by erythroblasts, erythroferrone (ERFE) was discovered by the Ganz lab (Kautz et al, 2014); ERFE suppresses hepcidin synthesis in hepatocytes, thus leading to increased Fe availability for the expanding erythroid marrow. ERFE levels were also found to be higher in blood donors and in mice subjected to hemorrhage or erythropoietin, consistent with appropriate Fe delivery to meet the needs of enhanced erythropoiesis. Several subsequent studies have also shown that in conditions associated with significant ineffective erythropoiesis such as β-thalassemia and dyserythropoietic anemias, ERFE expression is inappropriately increased, leading to the suppression of hepcidin synthesis and thus contributing to the worsening of Fe overload. We analyzed plasma hepcidin and ERFE levels in the same 22 SCD patients with Fe overload, and 14 SCD controls without Fe overload that we previously reported on (Mangaonkar et al, 2014); mean age of Fe overloaded patients was 33.4 years, and that of SCD controls was 29.0. Plasma stored at -80°C was used for both hepcidin and ERFE assays. ERFE and hepcidin levels were measured using commercially available ELISA kits from Biomatik Inc., Canada and DRG International, Inc., USA respectively, according to manufacturer's instructions. Hepcidin levels were significantly higher (41.59 vs 14.1 ng/ml, p=0.0297) and ERFE significantly lower (3.72 vs 5.46 ng/ml, p=0.0065) in cases vs. controls. ERFE/hepcidin ratios were also significantly lower among cases compared to controls (0.29 vs 1.62, p=0.011). These results suggest that in Fe overloaded SCD patients, ERFE is appropriately downregulated leading to higher hepcidin levels thus restricting further Fe loading. This is in contrast to what has been reported in both transfused and non-transfused β-thalassemia patients, where ineffective erythropoiesis overrides the appropriate regulation of the ERFE/hepcidin axis, leading to high ERFE levels, and worsening Fe overload. We speculate that this may be one mechanism explaining the different severity of Fe overload between SCD and β-thalassemia patients.
Kutlar: BlueBird Bio: Other: Member of Data Monitoring Committee; Sancilio & Co (OMEG-411-02): Other: Chair of Data and Safety Monitoring Board; Reprixys Pharmaceuticals Corporation (formerly known as Selexys Pharmaceuticals Corporation, which is not affiliated with Selexis S.A.): Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal